

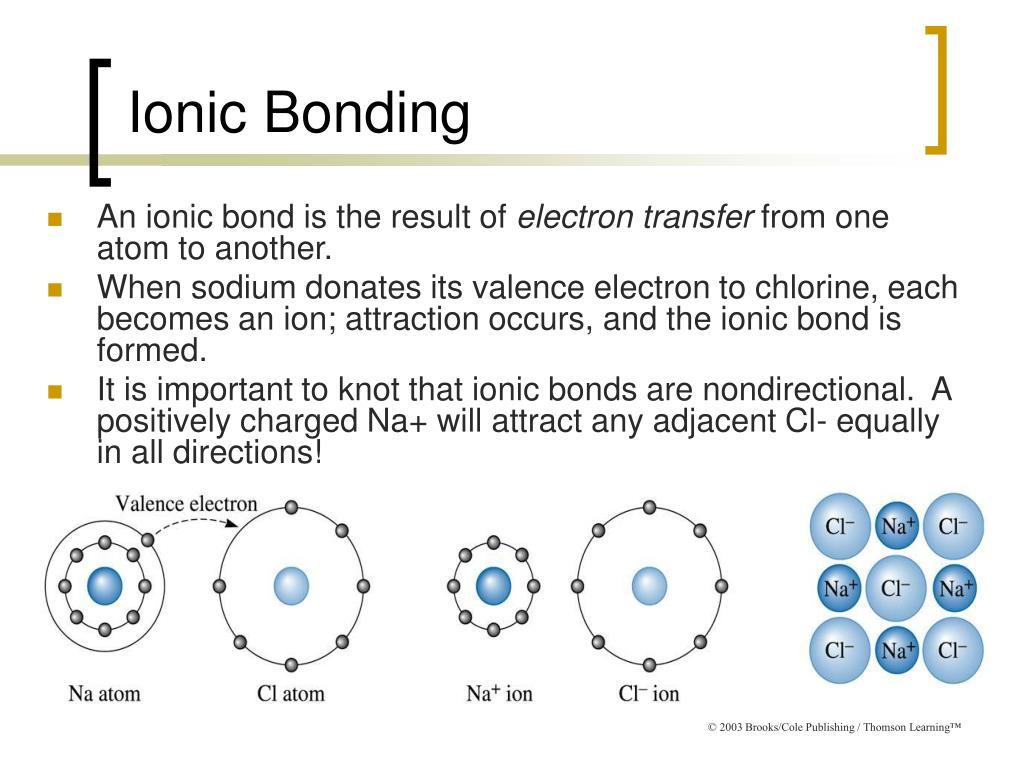
Negative Side Water – Durable to seal against hydrostatic pressure.Penetrates into most hard-troweled concrete surfaces. Highly Penetrates – Penetrates up to 1.5″ into concrete three times deeper than typical siloxane sealers.Waterproofs – Indoor/Outdoor Concrete, Brick, Porous Masonry.Use Ion-Bond Armor on concrete blocks and cinder blocks after sealing them first with RadonSeal Plus.īefore installing flooring on concrete slabs, use Ion-Bond Armor after sealing first with RadonSeal for the tightest possible seal against water vapor. The only silane/siloxane sealer able to withstand negative-side hydrostatic pressure. Use for waterproofing basements, foundation slabs, retaining walls, outdoor concrete, bricks, and pavers. Smooth Concrete Does Not Require Etching. PAINTED & SMOOTH CONCRETE – So Penetrating That It Even Waterproofs Through a Single Layer of Latex Paint.PAINTABLE – Leaves Surface Suitable for Paints, Adhesives, or Thinset.PERMANENT – No Re-Applications Needed.PREMIUM-GRADE CHEMISTRY – The Only Siloxane Able to Seal Against Negative Side Water Pressure.PENETRATES Up To 2″ Into Concrete, Brick, or Masonry (3 x deeper than a typical siloxane sealer) Chemically reacts to form a hydrophobic barrier deep inside the substrate. Penetrates up to 1.5 inches into concrete (3 times deeper than other siloxane sealers). Little bit more specific, a positive ion is called a cation and a negative ion is called an anion.Ion-Bond Armor is a subsurface elastomeric sealer for concrete, brick, and masonry. But as soon as we become non-neutral, we have either moreĮlectrons or more protons, and this is true of an atom or a molecule, we will then call it an ion. Say atom when we have the same number of electrons and protons, that's when we are neutral. let's see if I subtract 70 I'll get 125, minus eight I have 117 neutrons. Sides and what do you get? The number of neutrons is equal to 1.
#Ion bonding utube video plus
But what about its neutrons? Well protons plus neutrons is going to be equal to our mass number. Neutrons does it contain and what is its charge? We figured out its charge. Has a mass number of 195 and contains 74 electrons. But we're not done answering the question. This is just to get ourselves used to some of the terminology. Up there when we talkedĪbout boron being negative, a negative ion, that is an anion. Talking about a positive ion, we're talking about a cation.
This is a platinum ion,Ī positive platinum ion. Four more of the positive thing than you have of the negative things. So you're going to haveĪ positive four charge. That we have four more protons than electrons.

So by definition platinum hasħ8 protons, so we know that. Neutrons does it contain and what is its charge? Alright, so let's thinkĪbout this a little bit. And 195 looks prettyĬlose to that atomic mass we have there. An atom of platinum hasĪ mass number of 195. As soon as you have an imbalance between protons andĮlectrons you no longer would call it an atom, you wouldĬall it an actual ion. So you can write it like this, one minus. But this one has one extra electron, so it has one extra negative charge. The other way around? What if you were to have five protons, five protons and six electrons? What would this be? Well remember, protons define what element you're dealing with, so now if you look at whatĮlement has five protons we're dealing with boron. This you would now call an ion because it has that net charge. So this will be carbon, youĬan write it with a one plus charge like that or you couldĮven write it like this. So we're still dealing with carbon, but now we have one more positive charge than we have a negative charge. What define what element we're dealing with. So for example, if you had six protons and five electrons what would that be? Well, we still have six protons.

Is if you don't have an equal amount of protons and electrons. Now you could have a carbon ion, although they aren't that typical. You have the six positive charges and the six negative charges. It is going to have six electrons and that's what makes it neutral. And if it is neutral carbon it is going to have the That one atom of carbon? Well, by definition an atom For example, if I haveĬarbon, carbon is an element. So before we talk about ions we're just going to talkĪbout the idea of an element. Talk about in this video is the notion of an ion.


 0 kommentar(er)
0 kommentar(er)
